INTRODUCTION
Human epidermal growth factor receptor 2 (HER2) is overexpressed on the cell surface and/or there is gene amplification in approximately 20% of all breast cancers.[1] As a result, approximately 45,000-46,000 American women develop HER2-positive breast cancer each year.[2,3] HER2 status has prognostic significance: HER2-positive tumors are more aggressive and, without specific therapy, are associated with worse outcomes.[4] However, in 2012 the most important role of HER2 is determination of eligibility for HER2-targeted therapy with trastuzumab or lapatinib.[1,5]
Although HER2-targeted therapy has improved outcomes in early breast cancer and in the metastatic setting, in the latter case it is typical to see progression after an initial response.[6,7] Therefore, research continues into optimizing the use of currently available agents and into novel agents including the novel HER2 antibody pertuzumab, the conjugate trastuzumab emtansine, and novel tyrosine kinase inhibitors such as afatinib and neratinib.
This monograph reviews the rationale for these new approaches and novel agents—including the antibody pertuzumab, recently approved by the US Food and Drug Administration (FDA)—and the clinical data to date.
HER2 SIGNALING AND CROSSTALK
HER2 is a member of the HER family of tyrosine kinase cell surface receptors, also called ErbB. They are named HER1/ErbB1 (or epidermal growth factor receptor [EGFR]), HER2/ErbB2, HER3/ErbB3, and HER4/ErbB4.[8] A number of ligands have been identified that bind to HER1, HER3, and HER4, including EGF, transforming growth factor alpha (TGF-α), and the "regulins" such as amphiregulin, epiregulin, and neuregulin-1.[8,9] Binding of the ligand to the cell surface receptor induces conformational changes to that receptor, and dimers are formed. This in turn activates the associated tyrosine kinase and, depending on the receptor, downstream signaling pathways such as the Ras-Raf-MAPK (mitogen-associated protein kinase) pathway, phosphoinositide 3-kinase (PI3K)-AKT pathway, and protein kinase C (PKC) (Figure 1).[9]
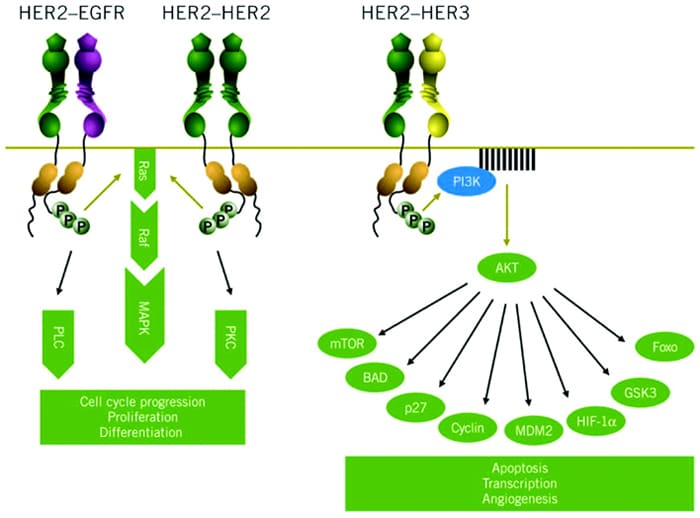
Figure 1. Signaling pathways activated by HER2.[9]
HER2 is unusual among the HER family in that it has no known ligands. Instead, HER2 is the preferred dimerization partner for other members of the family and appears to act as a coreceptor to improve ligand binding and signal transduction.[10] As such, HER2 can form heterodimers with other members of the HER family and can also form homodimers (ie, HER2/HER2 dimers; see Figure 1).[9]
HER2-mediated signaling is associated with cell proliferation, survival, and motility.[11] In HER2-postive cells these growth signals are thought to be driven mainly through HER2/HER3 heterodimers. Because HER3 lacks an associated tyrosine kinase, it requires HER2 for signal transduction, which results in activation of the PI3K-AKT pathway (Figure 1).[9,12] HER2 homodimers activate the Ras-Raf pathway, and it has been shown that the monoclonal antibody trastuzumab preferentially inhibits these HER2 homodimers, which has implications for both efficacy and resistance.[13]
ESTABLISHED HER2-TARGETED THERAPY
Two HER2-targeted therapies are currently available for the management of breast cancer: the immunoglobulin G1 (IgG1) antibody trastuzumab and the tyrosine kinase inhibitor lapatinib.[7,11] Trastuzumab binds to the extracellular HER2 receptor, where it disrupts HER2 signaling, downregulates HER2 expression, inhibits cell cycle progression, and induces antibody-dependent cell-mediated cytotoxicity (ADCC).[14,15] In addition, trastuzumab inhibits HER2 shedding,[16] which reduces levels of p95-HER2.[15] Lapatinib is a small-molecule inhibitor of the tyrosine kinase associated with HER2. It prevents phosphorylation and thereby inhibits downstream signaling. Lapatinib also inhibits the tyrosine kinase associated with HER1 (EGFR).[17]
Trastuzumab is FDA-approved for use in the adjuvant setting and also in patients with advanced/metastatic breast cancer.[18]It is also FDA-approved for gastric cancer,[18] which will not be discussed in detail within this activity. Lapatinib is FDA-approved for patients with advanced/metastatic breast cancer.[19]
Adjuvant use of trastuzumab in combination with cytotoxic chemotherapy is recommended for patients with HER2-positive, node-positive disease, based on the results from 5 pivotal studies.[5] These trials are summarized in Figure 2 and illustrate significant benefits in terms of progression-free survival (PFS) and, in some trials, overall survival (OS).[20-25] Although the FinHer trial utilized just 9 weeks of trastuzumab,[24] based on the results of the other 4 studies 1 year of adjuvant trastuzumab is the recommended duration.[5]
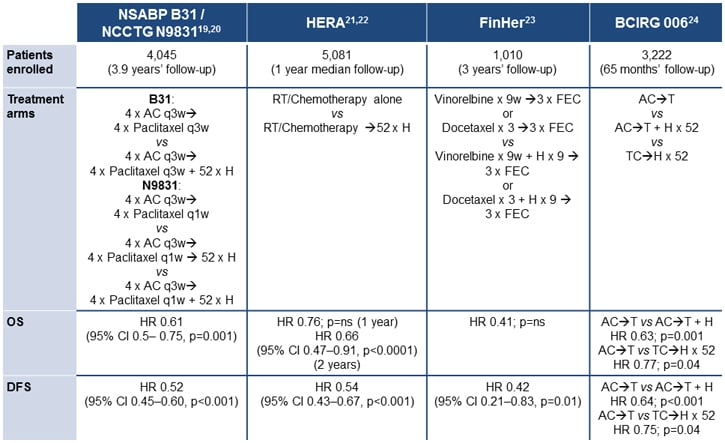
Figure 2. Adjuvant trastuzumab: selected studies.[20-25]
The TEACH (Tykerb Evaluation After Chemotherapy) study assessed the effect of delayed lapatinib in patients with HER2-positive breast cancer who had completed adjuvant chemotherapy but not received trastuzumab (eg, due to completion of chemotherapy before trastuzumab was available).[26] Patients had a median time between diagnosis and study entry of 2.7 years, and after 4 years' follow-up, no benefit in terms of PFS was detected (hazard ratio [HR], 0.83; 95% confidence interval [CI], 0.70-1.00; P = .053). However, in a preplanned subgroup analysis, significant benefit from lapatinib was observed in patients with hormone-receptor-negative disease (HR, 0.68; 95% CI, 0.52-0.89) and in those randomized within 1 year of diagnosis (HR, 0.70; 95% CI, 0.50-0.99), particularly in those individuals with tumors that were confirmed HER2-positive by central review.[26,27] The ALTTO (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) study is an ongoing trial, originally of 4 arms, comparing lapatinib alone, trastuzumab alone, the sequence (trastuzumab for 12 weeks followed by lapatinib), and the combination, as adjuvant therapy after chemotherapy.[11,28] More than 8000 patients have been enrolled; however, an interim analysis has indicated that the lapatinib-alone arm is not likely to meet the prespecified endpoint of noninferiority to trastuzumab alone, and this arm has been discontinued.
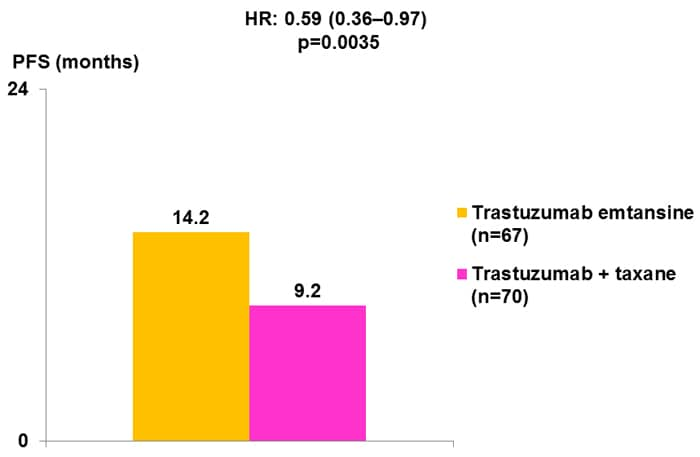
In addition, both trastuzumab and lapatinib are recommended in patients with advanced or metastatic HER2-positive breast cancer, with lapatinib recommended by the National Comprehensive Cancer Network (NCCN) for patients who progress after trastuzumab therapy.[5] Studies have demonstrated benefits from trastuzumab as monotherapy[29]; however, it is more usual to use trastuzumab in combination with cytotoxic chemotherapy.[5] A number of agents are recommended as suitable for use with trastuzumab, including paclitaxel, paclitaxel with carboplatin, docetaxel, vinorelbine, and capecitabine (results from selected studies are shown in Figure 3).[30-34]
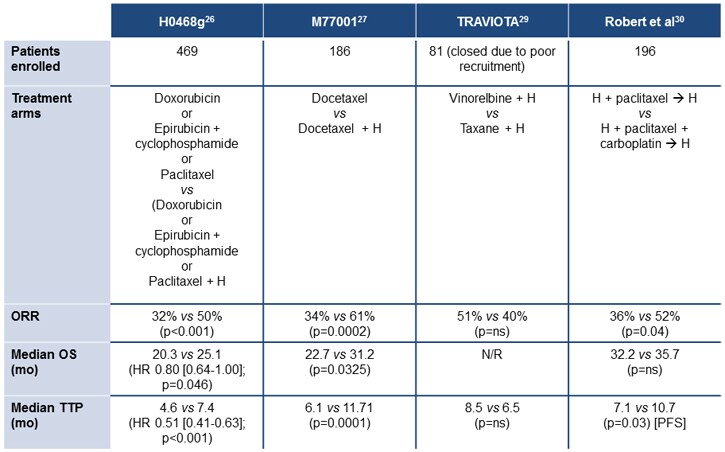
Figure 3. Trastuzumab in advanced/metastatic breast cancer.[30,31,33,34]
At the 2012 annual meeting of the American Society of Clinical Oncology (ASCO®), results were reported from the National Cancer Institute of Canada Clinical Trials Group's NCIC CTG MA.31 study, which compared first-line trastuzumab and lapatinib in patients with metastatic breast cancer. A total of 652 patients were enrolled, with data from 636 available (including 525 with HER2 status confirmed by central laboratory). Randomization was to taxane therapy (weekly paclitaxel or docetaxel) for 24 weeks in combination with either lapatinib or trastuzumab, followed by HER2-targeted therapy until progression.[35]After a median 13.6 months' follow-up, lapatinib was associated with inferior PFS compared with trastuzumab (8.8 months vs 11.4 months; HR, 1.33; 95% CI, 1.06-1.67; P = .01).
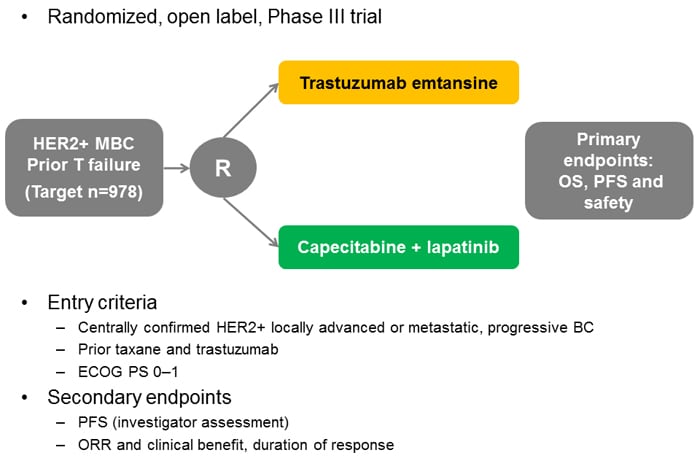
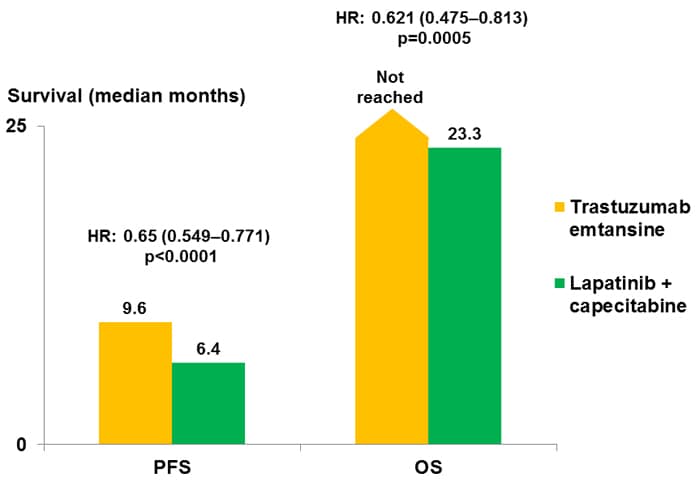
A phase 2 study of lapatinib in patients with previously treated metastatic or advanced breast cancer evaluated the HER2-positive cohort, all of whom had received trastuzumab, and reported objective responses in 4.3% of patients, with a median PFS of 9.1 weeks.[36] In contrast, there was no detectable benefit from lapatinib in HER2-negative patients, despite the activity of this compound against HER1. Following this and other phase 2 trials, the pivotal study compared capecitabine monotherapy with the combination of capecitabine and lapatinib in patients with trastuzumab-refractory advanced/metastatic disease.[37] This study reported significant benefits from the addition of lapatinib in terms of PFS (HR, 0.49; 95% CI, 0.34-0.71; P < .001), with a median time to progression of 8.4 months vs 4.4 months. Overall responses were reported in 22% of patients receiving lapatinib plus capecitabine, compared with 14% of those given capecitabine alone (P = not significant).
Benefits have also been reported for the addition of lapatinib to trastuzumab vs lapatinib alone in a chemotherapy-free regimen in patients with heavily pretreated metastatic breast cancer (Figure 4), indicating the benefits from "dual blockade" of the HER2 receptor.[38]
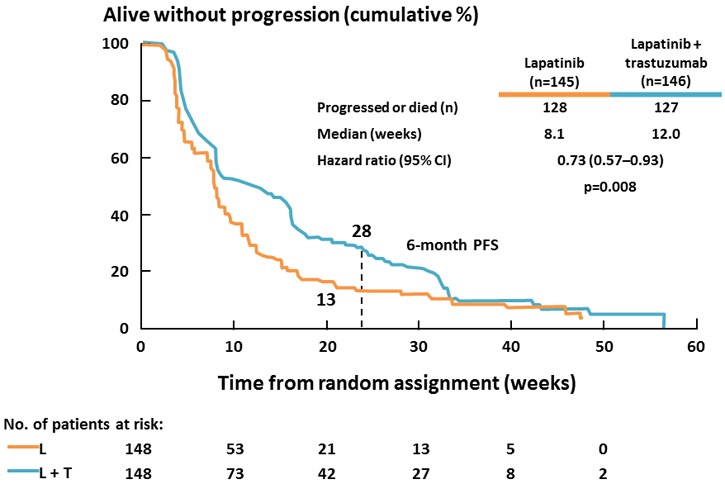
Figure 4. The combination of lapatinib and trastuzumab vs lapatinib alone in patients with trastuzumab-refractory metastatic breast cancer.[38]
Although not FDA-approved, studies have also assessed the effect of neoadjuvant trastuzumab added to paclitaxel-FEC (fluorouracil, epirubicin, and cyclophosphamide)[39] and to doxorubicin, paclitaxel, cyclophosphamide, methotrexate, and fluorouracil (the NOAH, or Neoadjuvant Herceptin, trial).[40] The GeparQuinto (GBG44) trial compared the addition of trastuzumab or lapatinib to neoadjuvant chemotherapy with epirubicin and cyclophosphamide followed by docetaxel (EC-T). Pathologic complete responses were reported significantly more frequently in the trastuzumab (EC-TH) group (93/307 evaluable; 30%) compared with the lapatinib (EC-TL) group (70/308; 23%) (P = .04). Although there was more edema and dyspnea in the EC-TH group, there was more diarrhea and skin rash in the EC-TL group, and more patients discontinued in the EC-TL arm (33% vs 14%).[41] The CHER-LOB (Chemotherapy, Herceptin and Lapatinib in Operable Breast Cancer ) study randomized 121 patients to paclitaxel for 12 weeks followed by 4 courses of FEC with lapatinib, trastuzumab, or the combination. Pathologic complete responses were seen significantly more frequently with the combination of trastuzumab and lapatinib (46.7%) than with either agent alone (25% and 26.3%; P = .019).[42] More recently, the combination of lapatinib and trastuzumab was also found to be more effective than either agent alone when added to neoadjuvant doxorubicin and cyclophosphamide followed by weekly paclitaxel (AC-T) chemotherapy, with pathologic complete responses in 62% of patients, compared with 52.5% for AC-T-trastuzumab and 53.2% for AC-T-lapatinib (P = .075).[43]
The phase 3 NeoALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) trial was a comparison of lapatinib, trastuzumab, and the combination for 6 weeks followed by a further 12 weeks of weekly paclitaxel before surgery in 455 patients with treatment-naïve, operable breast cancers greater than 2 cm in diameter. Adjuvant chemotherapy was combined with the same HER2-targeted therapy used prior to surgery.[44] This study also reported significantly more pathologic complete responses with the combined HER2 blockade, with no difference between the 2 single-agent HER2-targeted therapy arms (Figure 5). Grade 3 diarrhea was more common in lapatinib-treated patients (23% for monotherapy, 21% for the combination) than with trastuzumab (2%), and grade 3 liver enzyme abnormalities also occurred more frequently with lapatinib (17.5%) and lapatinib/trastuzumab (9.9%) than with trastuzumab (7.4%).[44]
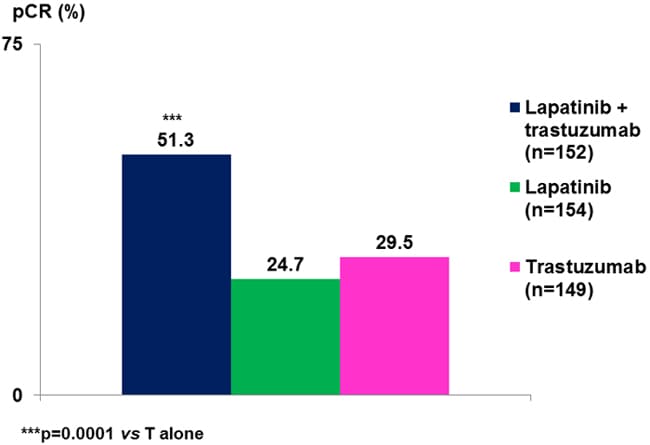
Figure 5. NeoALTTO: pathologic complete response rate increased with dual HER2 blockade.[44]
RESISTANCE MECHANISMS: HER2-TARGETED AGENTS
Despite the clinical benefits reported with HER2-targeted therapy, both de novo and acquired resistance to trastuzumab and lapatinib has been reported.[15] In patients with metastatic breast cancer, loss of response to HER2-targeted therapy is the norm.
The effect of lapatinib in patients with trastuzumab-refractory disease and the relative benefits of dual inhibition with lapatinib and trastuzumab in this setting provide some insights into the potential mechanisms of this resistance. This is complemented by preclinical and laboratory analysis of downstream signaling pathways, which has confirmed that multiple mechanisms of resistance exist. These can be classified as barriers to trastuzumab binding, upregulation of downstream signaling, crosstalk, and failure to trigger ADCC (Figure 6).[15,45] The majority of these mechanisms also allow tumor cells to escape lapatinib, although activation of alternate tyrosine kinases is also an important mechanism.[46] Mutations in the HER2 gene appear to be rare, however,[47] unlike acquired resistance to HER1/EGFR tyrosine kinase inhibitors.
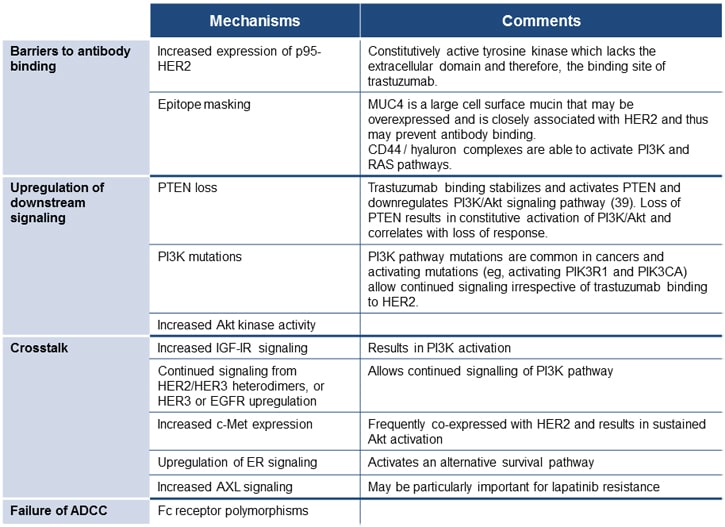
Figure 6. Mechanisms of resistance to HER2-targeted therapy.[15,45-47]
These findings have prompted research not only into alternative uses of the existing HER2-targeted therapy, but also into the combined use of trastuzumab and lapatinib to provide a more complete blockade of signaling. Dual blockade of HER2 by the combination of lapatinib and trastuzumab may, however, result in upregulation of the estrogen receptor, allowing it to function as an alternative survival pathway.[48] Therefore, novel agents that circumvent some of these mechanisms are also being actively studied. The most advanced of these agents are described in the next section.
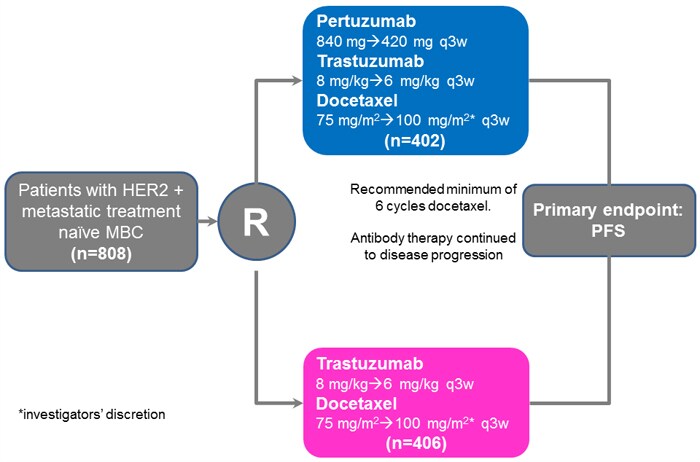
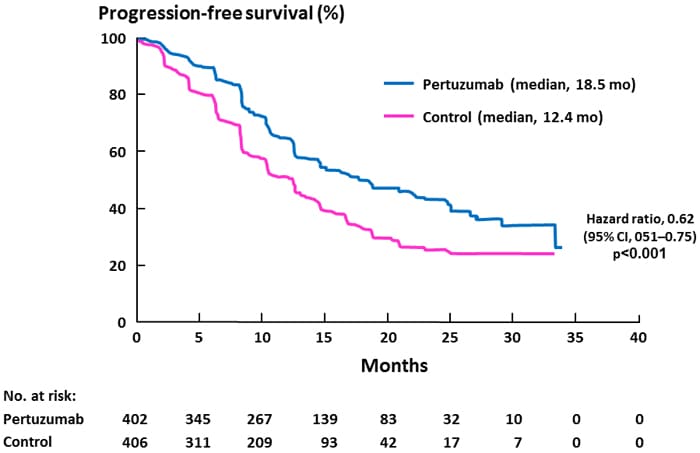
Comments
Post a Comment